CRISTALENS – Famiglia di Lenti Intraoculari ARTIS
Founded in 1994, CRISTALENS SA created its own production unit in Lannion, for hydrophilic and hydrophobic intraocular lenses for cataract and refractive surgery, becoming the first manufacturer of intraocular lenses in France. In 2008, CRISTALENS INDUSTRIE developed a new hydrophobic raw material that allows for micro-incisions under 2.0 mm.
MONOFOCAL HYDROPHOBIC LENS
Preloaded
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Lens Type | For implantation in the capsular bag |
| Optic diameter | 6.15 mm (from 0.0D to +9.5D) 6.00 mm (from +10.0D to +25.0D) 5.80 mm (from +25.5D to +35.0D) |
| Overall diameter | 11.00 mm (from 0.0D to +9.5D) 10.79 mm (from +10.0D to +25.0D) 10.50 mm (from +25.5D to +35.0D) |
| Design | One piece square edge on 360° |
| Optic design | Monofocal Aspherical with negative spherical aberration to partly correct corneal spherical aberration |
| Angulation | 5° |
| Material | Hydrophobic CBK 1.8 from Cristalens |
| Dioptric powers | From 0.0D to +35.0D by 0.5D |
| Estimated A-Constant (SRK-T) | 119.3 Ultrasound biometry 119.7 Interference laser biometry |
| Suggested Anterior Chamber Depth (ACD) |
5.77 mm Ultrasound biometry 6.03 mm Interference laser biometry |
| Refractive index | 1.54 |
| Sterilization | Ethylene oxide |
| Injection system | Preloaded system |
| Recommended incision size | 2.0 mm |
MONOFOCAL HYDROPHOBIC LENS
Preloaded
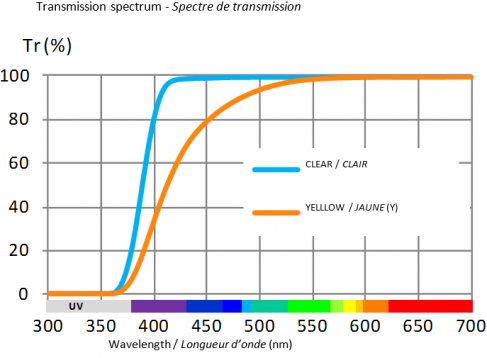
Natural yellow filter
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Lens Type | For implantation in the capsular bag |
| Optic Diameter | 6.15 mm (from 0.0D to +9.5D) 6.00 mm (from +10.0D to +25.0D) 5.80 mm (from +25.5D to +35.0D) |
| Overall diameter | 11.00 mm (from 0.0D to +9.5D) 10.79 mm (from +10.0D to +25.0D) 10.50 mm (from +25.5D to +35.0D) |
| Design | One piece square edge on 360° |
| Optic design | Monofocal Aspherical with negative spherical aberration to partly correct corneal spherical aberration |
| Angulation | 5° |
| Material | Hydrophobic CBJ 1.8 from Cristalens |
| Dioptric powers | From 0.0D to +35.0D by 0.5D |
| Estimated A-Constant (SRK-T) | 119.3 Ultrasound biometry 119.7 Interference laser biometry |
| Suggested Anterior Chamber Depth (ACD) |
5.77 mm Ultrasound biometry 6.03 mm Interference laser biometry |
| Refractive index | 1.54 |
| Sterilization | Ethylene oxide |
| Injection system | Preloaded system |
| Recommended incision size | 2.0 mm |
MONOFOCAL TORIC HYDROPHOBIC LENS
Preloaded
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Lens type | For implantation in the capsular bag |
| Optic diameter | 6.00 mm (from +10.0D to +25.0D) 5.80 mm (from +25.5D to +35.0D) |
| Overall diameter | 10.79 mm (from +10.0D to +25.0D) 10.50 mm (from +25.5D to +35.0D) |
| Design | One piece square edge on 360° |
| Optic design | Monofocal Aspherical with negative spherical aberration to partly correct corneal spherical aberration Toricity and marks on the posterior surface, biconvex |
| Angulation | 5° |
| Material | Hydrophobic CBK 1.8 from Cristalens |
| Dioptric powers (spherical equivalent) |
From +10.0D to +35.0D by 0.5D |
| Cylinder powers | +0.75D / +1.50D / +2.25D / +3.00D +3.75D / +4.50D / +5.25D / +6.00D |
| Estimated A-Constant (SRK-T) | 119.3 Ultrasound biometry 119.7 Interference laser biometry |
| Suggested anterior chamber depth (ACD) |
5.77 mm Ultrasound biometry 6.03 mm Interference laser biometry |
| Refractive index | 1.54 |
| Injection system | Preloaded system |
| Sterilization | Ethylene oxide |
| Recommended incision size | 2.0 mm |
MULTIFOCAL HYDROPHOBIC LENSES
Preloaded

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No [960783]
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Lens type | For implantation in the capsular bag |
| Optic diameter | 6.00 mm |
| Overall diameter | 10.79 mm |
| Design | One-piece square edge on 360° |
| Optic design | Diffractive multifocal extended depth of focus with binocular complementarity Aspherical with negative spherical aberration to partly correct corneal spherical aberration Diffractive pattern on the anterior face, biconvex |
| Angulation | 5° |
| Material | Hydrophobic CBK 1.8 from Cristalens |
| Dioptric powers | From +10.0D to +35.0D by 0.5D |
| Additions (at IOL plane) | MID: Superior intermediate vision – PLUS: Superior near vision |
| Estimated A-Constant (SRK-T) | 119.3 Ultrasound biometry 119.7 Interference laser biometry |
| Suggested anterior chamber depth (ACD) | 5.77 mm Ultrasound biometry 6.03 mm Interference laser biometry |
| Refractive index | 1.54 |
| Sterilization | Ethylene oxide |
| Injection system | Preloaded system |
| Recommended incision size | 2.0 mm |
TORIC MULTIFOCAL
HYDROPHOBIC LENSES
Preloaded
 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No [960783]
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No [960783]
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Lens type | For implantation in the capsular bag |
| Optic diameter | 6.00 mm |
| Overall diameter | 10.79 mm |
| Design | One-piece square edge on 360° |
| Optic design | Diffractive multifocal extended depth of focus with binocular complementarity Aspherical with negative spherical aberration to partly correct corneal spherical aberration Diffractive pattern on the anterior face, toxicity and marks on the posterior face, biconvex |
| Angulation | 5° |
| Material | Hydrophobic CBK 1.8 from Cristalens |
| Dioptric powers (spherical equivalent) |
From +10.0D to +35.0D by 0.5D |
| Additions (at IOL plane) | MID: Superior intermediate vision – PLUS: Superior near vision |
| Cylinder powers | +0.75D / +1.50D (+2.25D / +3.00D / +3.75D available upon request) |
| Estimated A-Constant (SRK-T) | 119.3 Ultrasound biometry 119.7 Interference laser biometry |
| Suggested anterior chamber depth (ACD) |
5.77 mm Ultrasound biometry 6.03 mm Interference laser biometry |
| Refractive index | 1.54 |
| Sterilization | Ethylene oxide |
| Injection system | Preloaded system |
| Recommended incision size | 2.0 mm |